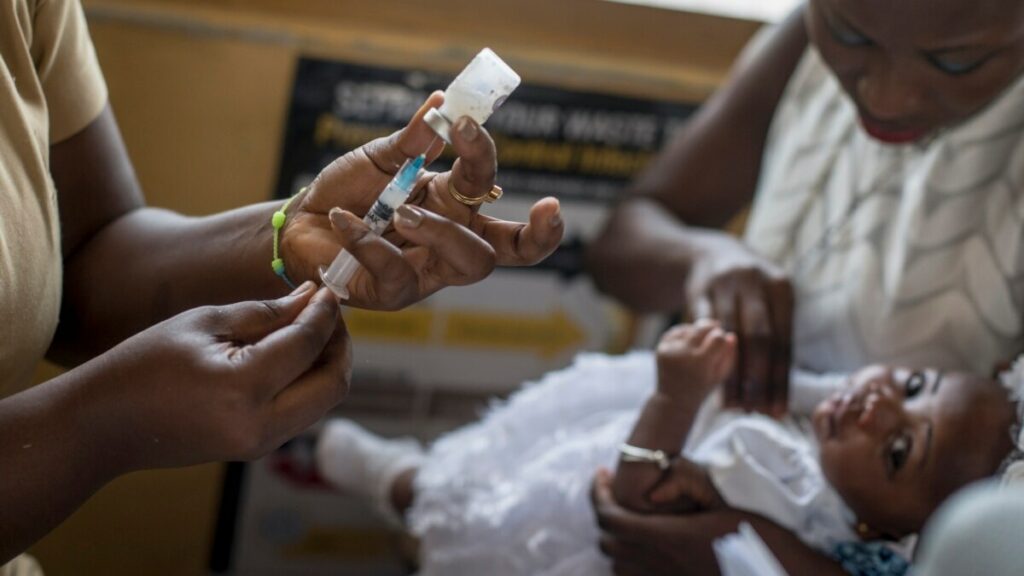
A malaria vaccine developed by Britain’s Oxford University has been cleared for use in Ghana. It is the first time the shot has received regulatory approval anywhere in the world.
Read More: Antonio Guterres Appeals for Int’l Support for Somalia
“The vaccine has been approved for use in children aged 5-36 months, the age group at highest risk of death from malaria,” the university said in a statement.
It is hoped that this first crucial step will enable the vaccine to help Ghanaian and African children to effectively combat malaria. Professor Adrian Hill, chief investigator of the R21/Matrix-M vaccine program and director of the university’s Jenner Institute, said it marked the “culmination of 30 years of malaria vaccine research at Oxford with the design and provision of a high efficacy vaccine that can be supplied at adequate scale to the countries who need it most.”
The effort is one of several focused on addressing the disease that kills over 600,000 each year, most of them children in Africa. The complicated structure and life cycle of the malaria parasite has long stymied efforts to develop vaccines.
After decades of work, the first malaria vaccine, Mosquirix from British drugmaker GSK, was last year endorsed by the World Health Organization (WHO). But a lack of funding and commercial potential has thwarted the company’s capacity to produce enough doses.
Read More: Impact of the new Immigration regulations on the daily life of migrants in Spain
The GSK vaccine has been administered to more than a million children in Africa so far. Research shows it has an effectiveness of around 60%, but that protection wanes significantly over time. The Oxford vaccine has a manufacturing advantage, thanks to a deal with Serum Institute of India to produce up to 200 million doses annually.
In contrast, GSK has committed to producing up to 15 million doses of Mosquirix every year through 2028, well under the roughly 100 million doses a year of the four-dose vaccine the WHO says is needed long-term to cover around 25 million children.
Mid-stage data from the Oxford vaccine trial involving more than 400 young children was published in a medical journal in September.
Vaccine effectiveness was 80% in the group that received a higher dose of the immune-boosting adjuvant component of the shot, and 70% in the lower-dose adjuvant group, at 12 months following the fourth dose. The doses were administered ahead of the peak malaria season in Burkina Faso.
Read More: Zanzibar Eyes to Attract More Tourists From Belgium
Data from an ongoing phase III clinical trial in Burkina Faso, Kenya, Mali and Tanzania that has enrolled 4,800 children is expected to be published in a medical journal in the coming months.